Contact WorkSafe
Tel: 1300 307 877
Email us
24 hour serious incident and fatality reporting line
Freecall: 1800 678 198
Mason Bird Building
303 Sevenoaks St
Cannington WA 6107
View on Google Maps
The GHS is a system used to classify and communicate chemical hazards using internationally consistent terms and information on chemical labels and safety data sheets. The system was developed by the United Nations with the intention of harmonising the many different chemical classification systems in use around the world.
The GHS provides criteria for the classification of physical hazards (e.g. flammable liquids), health hazards (e.g. carcinogens) and environmental hazards (e.g. aquatic toxicity).
Western Australia has included use of the GHS (edition 7) as part of the Work Health and Safety (General) Regulations 2022 and the Work Health and Safety (Mines) Regulations 2022, and therefore chemical classification and labelling is required to be GHS compliant.
No. When transporting dangerous goods, it is a requirement to comply with the Australian Dangerous Goods Code (ADG Code). You must continue to comply with the ADG Code and relevant State and Territory dangerous goods transport laws for road and rail.
The GHS introduces different classifications and labelling in the following ways:
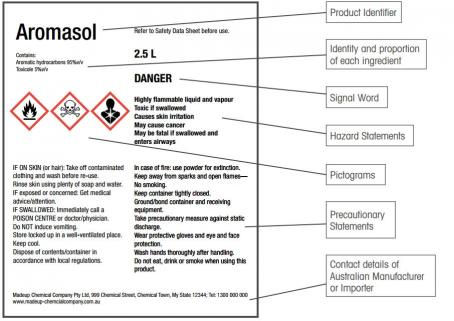
Signal words
Either Danger or Warning to describe the hazard level.
Hazard statement
The hazard statement is a description of the chemical hazard assigned to a particular hazard category, for example ‘highly flammable liquid and vapour’ or ‘causes skin irritation’.
Precautionary statement
Precautionary statements recommend actions to take to reduce the risk of chemical exposure. These phrases are specific to prevention, storage, disposal and response, for example ‘keep away from heat/sparks/open flames/hot surfaces – no smoking’, ‘wear protective gloves/eye protection/face protection’ or ‘store in a well-ventilated space’.
Pictograms
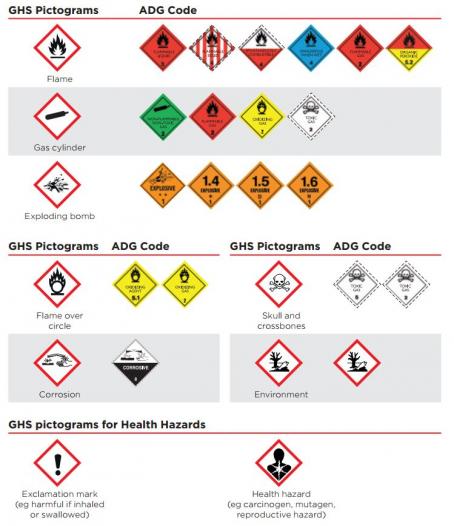
There are nine hazard pictograms in the GHS:

Where there is an equivalent ADG dangerous goods pictogram available, that is an acceptable alternative to a pictogram. The table below compares GHS hazard pictograms with the corresponding ADG Code labels.
Special labelling situations
Pesticide and veterinary medicine labels (other than some Schedule 4 and Schedule 8 veterinary medicines) have also introduced GHS based information.
It is important to note that pesticides and veterinary medicines in Australia go through a rigorous risk assessment process and are registered via the Australian Pesticides and Veterinary Medicines Authority (APVMA) before they can be used. All necessary information and controls to reduce risks to workers, the public, industry and the environment are included on the label.
The GHS labelling system, now included on labels in relation to hazards, is hazard based – that is, hazard information is included on the label whether the hazard has been addressed under the APVMA’s risk assessment process or not.
Hazard based labelling systems have been used for other workplace chemicals and included on safety data sheets for pesticides and veterinary medicines for many years.
If the existing risk based phrase is similar to the hazard based phrase the change may only include the addition of a hazard statement. For example, the warning on a herbicide label may change from:

This change of approach means you see more safety and health warnings on some new pesticide and veterinary medicine labels (including warnings about chronic health hazards such as cancer); however it doesn’t mean that the pesticide or veterinary medicine is more hazardous than previously thought. GHS hazard phrases relate to hazards to the user of the chemical product as supplied, rather than to consumers of end products such as food crops or animals. Any hazards to consumers are considered as part of the APVMA’s risk assessment.
Consumer products and therapeutic goods require labelling under the Poisons Standard and the Therapeutic Goods Act 1989. Chemicals which are used in workplaces in quantities and ways that are consistent with household use, and are used in a way that is incidental to the work that is being carried out, do not need to be labelled in accordance with the GHS.
Therapeutic goods are exempt from workplace labelling when in a form and package intended for intake or administration to a patient or consumers, or intended for use for therapeutic purposes.
Veterinary medicines that are labelled in accordance with the Australian Pesticides and Veterinary Medicines Authority and are listed in either Schedule 8 or Schedule 4 (in the form and packaging consistent with direct administration to animals) are exempt from the labelling requirements of the GHS.
Last modified: